
Telomeres, the repetitive sequences of DNA at the ends of linear chromosomes, have an important function: They protect vulnerable chromosome ends from molecular attack. Researchers at Rockefeller University now show that telomeres have their own weakness. They resemble unstable parts of the genome called fragile sites where DNA replication can stall and go awry. But what keeps our fragile telomeres from falling apart is a protein that ensures the smooth progression of DNA replication to the end of a chromosome.The research, led by Titia de Lange, head of the Laboratory of Cell Biology and Genetics, and first author Agnel Sfeir, a postdoctoral associate in the lab, suggests a striking similarity between telomeres and common fragile sites, parts of the genome where breaks tend to occur, albeit infrequently. (Humans have 80 common fragile sites, many of which have been linked to cancer.) De Lange and Sfeir found that these newly discovered fragile sites make it difficult for DNA replication to proceed, a discovery that unveils a new replication problem posed by telomeres.
At the center of the discovery is a protein known as TRF1, which de Lange, in an effort to understand how telomeres protect chromosome ends, discovered in 1995. Using a conditional mouse knockout, de Lange and Sfeir have now revealed that TRF1, which is part of a six-protein complex called shelterin, enables DNA replication to drive smoothly through telomeres with the aid of two other proteins.
“Telomeric DNA has a repetitive sequence that can form unusual DNA structures when the DNA is unwound during DNA replication,” says de Lange. “Our data suggest that TRF1 brings in two proteins that can take out these structures in the telomeric DNA. In other words, TRF1 and its helpers remove the bumps in the road so that the replication fork can drive through.”
The work, published in the July 10 issue of Cell, began when Sfeir deleted TRF1 and saw that the telomeres resembled common fragile sites, suggesting that TRF1 protects telomeres from becoming fragile. Instead of a continuous string of DNA, the telomeres were broken into fragments of twos and threes. To see if the replication fork stalls at telomeres, de Lange and Sfeir joined forces with Carl L. Schildkraut, a researcher at Albert Einstein College of Medicine in New York City. Using a technique called SMARD, the researchers observed the dynamics of replication across individual DNA molecules — the first time this technique has been used to study telomeres. In the absence of TRF1, the fork often stalled for a considerable amount of time.
The only other known replication problem posed by telomeres was solved in 1985 when it was shown that the enzyme telomerase elongates telomeres, which shorten during every cell division. The second problem posed by telomeres, the so-called end-protection problem, was solved by de Lange and her colleagues when they found that shelterin protects the ends of linear chromosomes, which look like damaged DNA, from unnecessary repair. Working with TRF1, the very first shelterin protein ever to be identified, de Lange and Sfeir have not only unveiled a completely unanticipated replication problem at telomeres, they have also shown how it is solved.
The research lays new groundwork for the study of common fragile sites throughout the genome, explains de Lange. “Fragile sites have always been hard to study because no specific DNA sequence preceeds or follows them,” she says. “In constrast, telomeres represent fragile sites with a known sequence, which may help us understand how common fragile sites break throughout the genome — and why.”
INTERNAL MEDICINE The internal medicine blog , where you can have details on alternatice medicine , latest trends in medicine , new drugs in the market , school of medicine etc
Handle With Care: Telomeres Resemble DNA Fragile Sites
Friday, July 17, 2009 at 6:34 AM Posted by Sajith
HIV-related Death: Predicting Fatal Fungal Infections
Saturday, July 4, 2009 at 4:22 AM Posted by Sajith
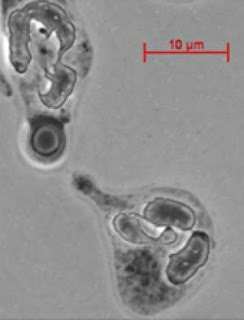
In a study published in The Journal of Infectious Diseases, researchers from Albert Einstein College of Medicine of Yeshiva University have identified cells in blood that predict which HIV-positive individuals are most likely to develop deadly fungal meningitis, a major cause of HIV-related death. This form of meningitis affects more than 900,000 HIV-infected people globally—most of them in sub-Saharan Africa and other areas of the world where antiretroviral therapy for HIV is not available.A major cause of fungal meningitis is Cryptococcus neoformans, a yeast-like fungus commonly found in soil and in bird droppings. Virtually everyone has been infected with Cryptococcus neoformans, but a healthy immune system keeps the infection from ever causing disease.
The risk of developing fungal meningitis from Cryptococcus neoformans rises dramatically when people have weakened immunity, due to HIV infection or other reasons including the use of immunosuppressive drugs after organ transplantation, or for treating autoimmune diseases or cancer. Knowing which patients are most likely to develop fungal meningitis would allow costly drugs for preventing fungal disease to be targeted to those most in need. (In the U.S., the widespread use of antiretroviral therapy by HIV-infected people, and their preventive use of anti-fungal drugs, has dramatically reduced their rate of fungal meningitis from Cryptococcus neoformans to about 2%.)
In this study, Liise-anne Pirofski, M.D., describes a technique for predicting which HIV-infected patients are at greatest risk for developing fungal meningitis caused by Cryptococcus neoformans. Dr. Pirofski is chief in the division of infectious diseases at Einstein.
Dr. Pirofski and her colleagues counted the number of immune cells known as IgM memory B cells in the bloodstream of three groups of individuals: people infected with HIV who had a history of fungal meningitis caused by Cryptococcus neoformans; people infected with HIV but with no history of the disease; and those with no history of either HIV infection or the disease.
"We were astounded to find a profound difference in the level of these IgM memory B cells between the HIV-infected groups," said Dr. Pirofski. "The HIV-infected people with fungal meningitis caused by Cryptococcus neoformans had much lower levels of these cells."
The research team wanted to know if the lower levels of IgM memory B cells in certain HIV-infected individuals resulted from the fungal disease, or whether their reduced levels of these cells preceded their development of the disease.
To find out, Dr. Pirofski analyzed frozen blood samples taken from HIV-infected patients before they had developed fungal meningitis due to Cryptococcus neoformans. Years before these HIV-infected patients were diagnosed with meningitis, their blood had far fewer IgM memory B cells than HIV-infected patients who didn't come down with the disease. This suggests that some people are predisposed to develop fungal meningitis because they have low levels of IgM memory B cells that may be due to their genetic makeup.
These findings could be important for many other immunocompromised patients in addition to those infected with HIV. "We think that knowing whether transplant recipients or other patients taking immunosuppressive drugs have low numbers of IgM memory B cells could be useful in deciding which patients should receive antifungal drugs to prevent meningitis caused by Cryptococcus neoformans," says Dr. Pirofski.
Krishanthi Subramanian, Ph.D., who did her thesis work in Dr. Pirofski's laboratory, is the first author of the study.
Police Work Undermines Cardiovascular Health, Comparison To General Population Shows
at 4:16 AM Posted by Sajith

It is well documented that police officers have a higher risk of developing heart disease: The question is why.In the most recent results coming out of one of the few long-term studies being conducted within this tightly knit society, University at Buffalo researchers have determined that underlying the higher incidence of subclinical atherosclerosis -- arterial thickening that precedes a heart attack or stroke -- may be the stress of police work.
"We took lifestyle factors that generally are associated with atherosclerosis, such as exercise, smoking, diet, etc., into account in our comparison between citizens and the police officers," said John Violanti, Ph.D., UB associate professor of social and preventive medicine, who has been studying the police force in Buffalo, N.Y., for 10 years.
"These lifestyle factors were statistically controlled for in the analysis. This led to the conclusion that it is not the 'usual' heart-disease-related risk factors that increase the risk in police officers. It is something else. We believe that 'something else' is the occupation of policing."
Results of the study appear in the June issue of the Journal of Occupational and Environmental Medicine.
Violanti and colleagues have been studying the role of cortisol, known as the "stress hormone," in these police officers to determine if stress is associated with physiological risk factors that can lead to serious health problems such as diabetes and cardiovascular disease.
In a study accepted for publication in Psychiatry Research that looked at the male-female differences in stress and signs of heart disease, Violanti found that female police officers had higher levels of cortisol when they awoke, and the levels remained high throughout the day. Cortisol normally is highest in the morning and decreases to its lowest point in the evening. The constantly high cortisol levels were associated with less arterial elasticity, a risk factor for heart disease, Violanti noted.
"When cortisol becomes dysregulated due to chronic stress, it opens a person to disease," he said. "The body becomes physiologically unbalanced, organs are attacked and the immune system is compromised as well. It's unfortunate, but that's what stress does to us."
In the current study, the researchers used carotid artery thickness to assess heart disease risk. Participants were 322 clinically healthy active-duty police officers from the Buffalo Cardio-Metabolic Occupational Police Stress (BCOPS) study and 318 healthy persons from the ongoing UB Western New York Health Study matched to the officers by age.
All measurements were taken in the morning after a 12-hour fast. In addition to testing carotid thickness via ultrasound, investigators measured blood pressure, body size, cholesterol (both total and HDL) and glucose. They collected information on physical activity, symptoms of depression, alcohol consumption and smoking history. These are the factors that typically cause heart disease.
Results showed that police work was associated with increased subclinical cardiovascular disease -- there was more plaque build-up in the carotid artery -- compared to the general population that could not be explained by those conventional heart disease risk factors.
Subclinical atherosclerosis means that the disease shows progression but does not qualify yet as overt heart disease.
"In this case we examined the thickness of the carotid artery as an indicator of increasing risk for atherosclerosis," noted Violanti. "The plaque buildup was greater in police than the citizen population.
"In future work, we will measure the carotid artery thickness again to see how much it has increased. At some point in time, the thickness may reach a stage of possible blockage, which will require medical intervention and treatment. We think that police officers will likely reach that stage quicker than the general population."
P. Nedra Joseph, Ph.D., a former postdoctoral researcher at UB, now at the Centers for Disease Control and Prevention (CDC), is first author on the study. Additional contributors to the study were: from UB -- Richard Donahue, Ph.D., and Joan Dorn, Ph.D., from the UB School of Public Health and Health Professions; Michael E. Andrew, Ph.D., and Cecil M. Burchfiel, from the CDC; and Maurizio Trevisan, M.D., formerly of UB, now head of the University of Nevada Health Sciences System.
The BCOPS study is funded by the National Institute for Occupational Safety and Health.
Search
Labels
- Alzheimer's (10)
- Antibiotics (7)
- Anxiety (1)
- articles (2)
- Bacteria (1)
- behavioral abnormality (1)
- Bio technology (1)
- Brain (8)
- breast cancer (6)
- Cancer (40)
- cancer treatment (7)
- chemotherapy (6)
- Chlamydia (1)
- Cytology (3)
- Death (1)
- Diabetes Mellitus (7)
- Diet (17)
- DNA (1)
- Doctors (1)
- epilepsy (2)
- Fossil (1)
- fruits (1)
- genes (6)
- Genetics (13)
- Geriatrics (1)
- Health (12)
- Heart diseases (9)
- Herbal Medicine (2)
- HIV and AIDS (7)
- influenza (1)
- Inventions (1)
- IVF (1)
- latest findings (1)
- Life style (2)
- Lung Cancer (1)
- Lung Disease (9)
- Microbiology (1)
- Multiple Sclerosis (1)
- Nanotechnology (3)
- Neonatology (1)
- neurology (1)
- News (18)
- Parkinsonism (2)
- Prevention (1)
- Primates (1)
- Prostate cancer (1)
- Prosthetics (1)
- seizure (2)
- Skin (1)
- STD (2)
- Stem cells (9)
- Stroke (1)
- Surgery (2)
- Swine flu (11)
- Virus (1)
- weight loss (1)
Search The Web
Minyx v2.0 template es un theme creado por Spiga. | Minyx Blogger Template distributed by eBlog Templates
