
A quick breath check in the palm of your hand can never give accurate results. Whether you're about to lean in for a smooch or start a job interview, you're better off asking a trusted friend if your breath is sweet. But what if a friend isn't around when you need one?
Tel Aviv University researchers have come up with the ultimate solution — a pocket-size breath test which lets you know if malodorous bacteria are brewing in your mouth. A blue result suggests you need a toothbrush. But if it's clear, you're "okay to kiss."
Until now, scientists believed that only one population of bacteria (the Gram-negative ones) break down the proteins in the mouth and produce foul odor. But Prof. Mel Rosenberg and Dr. Nir Sterer of TAU's Sackler Faculty of Medicine recently discovered that the other population of bacteria (the Gram-positive ones) are bad breath's bacterial partner. These bacteria appear to help the Gram-negative ones by producing enzymes that chop sugary bits off the proteins that make them more easily degraded. This enzymatic activity, present in saliva, serves as the basis for the new "OkayToKiss" test.
Prof. Rosenberg, international authority on the diagnosis and treatment of bad breath, who co-developed the kit with Dr. Sterer, published their findings this past March in the Journal of Breath Research, of which Prof. Rosenberg is editor-in-chief. An earlier invention of Prof. Rosenberg led to the development of two-phase mouthwashes that have become a hit in the UK, Israel and elsewhere.
From the Lab to Your Pocket
The patent-pending invention is the result of ongoing research in Prof. Rosenberg's laboratory.
"All a user has to do is dab a little bit of saliva onto a small window of the OkayToKiss kit," explains Prof. Rosenberg: "OkayToKiss will turn blue if a person has enzymes in the mouth produced by the Gram-positive bacteria. The presence of these enzymes means that the mouth is busily producing bacteria that foster nasty breath," he explains.
Apart from its social uses, the test can be used as an indicator of a person's oral hygiene, encouraging better health habits, such as flossing, brushing the teeth, or scheduling that long-delayed visit to the dentist.
OkayToKiss could be ready in about a year for commercial distribution, probably in the size of a pack of chewing gum, to fit in a pocket or purse. It is disposable and could be distributed alongside breath-controlling products.
Science Behind the Smells
"For about 7 years now, we've suspected that there's another kind of bacteria at work in the mouth which causes bad breath," says Prof. Rosenberg. "Now, we are able to grow these bacteria from saliva in an artificial biofilm, showing that there are two distinct populations at work."
In the biofilm — the basis of the new breath test — the color difference between the Gram-positive and Gram-negative bacteria is remarkable. In the top layer of the biofilm, the bacterium take the glycoproteins in the saliva and chop off sugar residues to produce volatile proteins. On the lower layer in the biofilm, which could roughly be compared to one's tongue or inner lining of the mouth, reside the known and established Gram-negative bacteria.
Biomarkers, like the ones used by Prof. Rosenberg's new invention, are the basis of popular diagnostic kits today, like home pregnancy tests or glucose monitors used by diabetics. Checking the sweetness of one's breath may seem frivolous, but millions worry about it on a continual basis. Prof. Rosenberg's continued research into biomarkers in saliva is very promising for diagnosing other more serious disorders, including indicators for lung cancer, asthma and ulcers.
Prof. Rosenberg has summarized his twenty years of his research and experience on bad breath problems in a new book, Save Your Breath, due out in two months. This new work is a collaborative effort with Dr. Nir Sterer and Dr. Miriam Shaharbany of the Sackler Medical Faculty.
INTERNAL MEDICINE The internal medicine blog , where you can have details on alternatice medicine , latest trends in medicine , new drugs in the market , school of medicine etc
Bad Breath? New Pocket-sized Breath Test Developed
Wednesday, May 20, 2009 at 7:34 AM Posted by Sajith
New Way Of Treating The Flu
at 7:30 AM Posted by Sajith
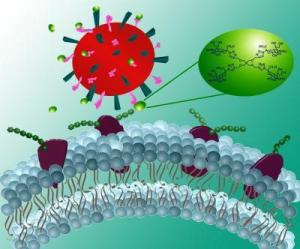
What happens if the next big influenza mutation proves resistant to the available anti-viral drugs? This question is presenting itself right now to scientists and health officials this week at the World Health Assembly in Geneva, Switzerland, as they continue to do battle with H1N1, the so-called swine flu, and prepare for the next iteration of the ever-changing flu virus.Promising new research announced by Rensselaer Polytechnic Institute could provide an entirely new tool to combat the flu. The discovery is a one-two punch against the illness that targets the illness on two fronts, going one critical step further than any currently available flu drug.
"We have been fortunate with H1N1 because it has been responding well to available drugs. But if the virus mutates substantially, the currently available drugs might be ineffective because they only target one portion of the virus," said Robert Linhardt, the Ann and John H. Broadbent Jr. '59 Senior Constellation Professor of Biocatalysis and Metabolic Engineering at Rensselaer. "By targeting both portions of the virus, the H and the N, we can interfere with both the initial attachment to the cell that is being infected and the release of the budding virus from the cell that has been affected."
The findings of the team, which have broad implications for future flu drugs, will be featured on the cover of the June edition of European Journal of Organic Chemistry.
The influenza A virus is classified based on the form of two of its outer proteins, hemagglutinin (H) and neuraminidase (N). Each classification – for example H5NI "bird flu" or H1N1 "swine flu" – represents a different mutation of hemagglutinin and neuraminidase or H and N.
Flu drugs currently on the market target only the neuraminidase proteins, and disrupt the ability of the virus to escape an infected cell and move elsewhere to infect other healthy cells. The new process developed by Linhardt is already showing strong binding potential to hemagglutinin, which binds to sialic acid on the surface of a healthy cell, allowing the virus to entire the cell.
"We are seeing promising preliminary results that the chemistry of this approach will be effective in blocking the hemagglutinin portion of the disease that is currently not targeted by any drug on the market," he said.
In addition, Linhardt and his team have shown their compound to be just as effective at targeting neuraminidase as the most popular drugs on the market, according to Linhardt.
The approach can also be modified to specifically target the neuraminidase or the hemagglutinin, or both, depending on the type of mutation that is present in the current version of the flu, according to Linhardt.
In the next steps of his research, Linhardt will look at how their compounds bind to hemagglutinin, and he will test the ability to block the virus first in cell cultures and then in infected animal models.
"It is still early in the process," he said. "We are several steps away from a new drug, but this technique is allowing us to move very quickly in creating and testing these compounds."
The technique that Linhardt used is the increasingly popular technique of "click chemistry." Linhardt is among the first researchers in the world to utilize the technique to create new anti-viral agents. The process allows chemists to join small units of a substance together quickly to create a new, full substance.
In this case, Linhardt used the technique to quickly build a new derivative of sialic acid. Because it is chemically very similar to the sialic acid found on the surface of a cell, the virus could mistake the compound as the real sialic acid and bind to it instead of the cell, eliminating the connections to hemagglutinin and neuraminidase that are required for initial infection and spread of the infection in the body. The currently available drugs are translation-state inhibitors whose chemical structure allows them to only effectively target the neuraminidase.
The research was funded by the National Institutes of Health. Linhardt was joined in the research by Michel Weïwer, Chi-Chang Chen, and Melissa Kemp of Rensselaer.
Search
Labels
- Alzheimer's (10)
- Antibiotics (7)
- Anxiety (1)
- articles (2)
- Bacteria (1)
- behavioral abnormality (1)
- Bio technology (1)
- Brain (8)
- breast cancer (6)
- Cancer (40)
- cancer treatment (7)
- chemotherapy (6)
- Chlamydia (1)
- Cytology (3)
- Death (1)
- Diabetes Mellitus (7)
- Diet (17)
- DNA (1)
- Doctors (1)
- epilepsy (2)
- Fossil (1)
- fruits (1)
- genes (6)
- Genetics (13)
- Geriatrics (1)
- Health (12)
- Heart diseases (9)
- Herbal Medicine (2)
- HIV and AIDS (7)
- influenza (1)
- Inventions (1)
- IVF (1)
- latest findings (1)
- Life style (2)
- Lung Cancer (1)
- Lung Disease (9)
- Microbiology (1)
- Multiple Sclerosis (1)
- Nanotechnology (3)
- Neonatology (1)
- neurology (1)
- News (18)
- Parkinsonism (2)
- Prevention (1)
- Primates (1)
- Prostate cancer (1)
- Prosthetics (1)
- seizure (2)
- Skin (1)
- STD (2)
- Stem cells (9)
- Stroke (1)
- Surgery (2)
- Swine flu (11)
- Virus (1)
- weight loss (1)
Search The Web
Minyx v2.0 template es un theme creado por Spiga. | Minyx Blogger Template distributed by eBlog Templates
